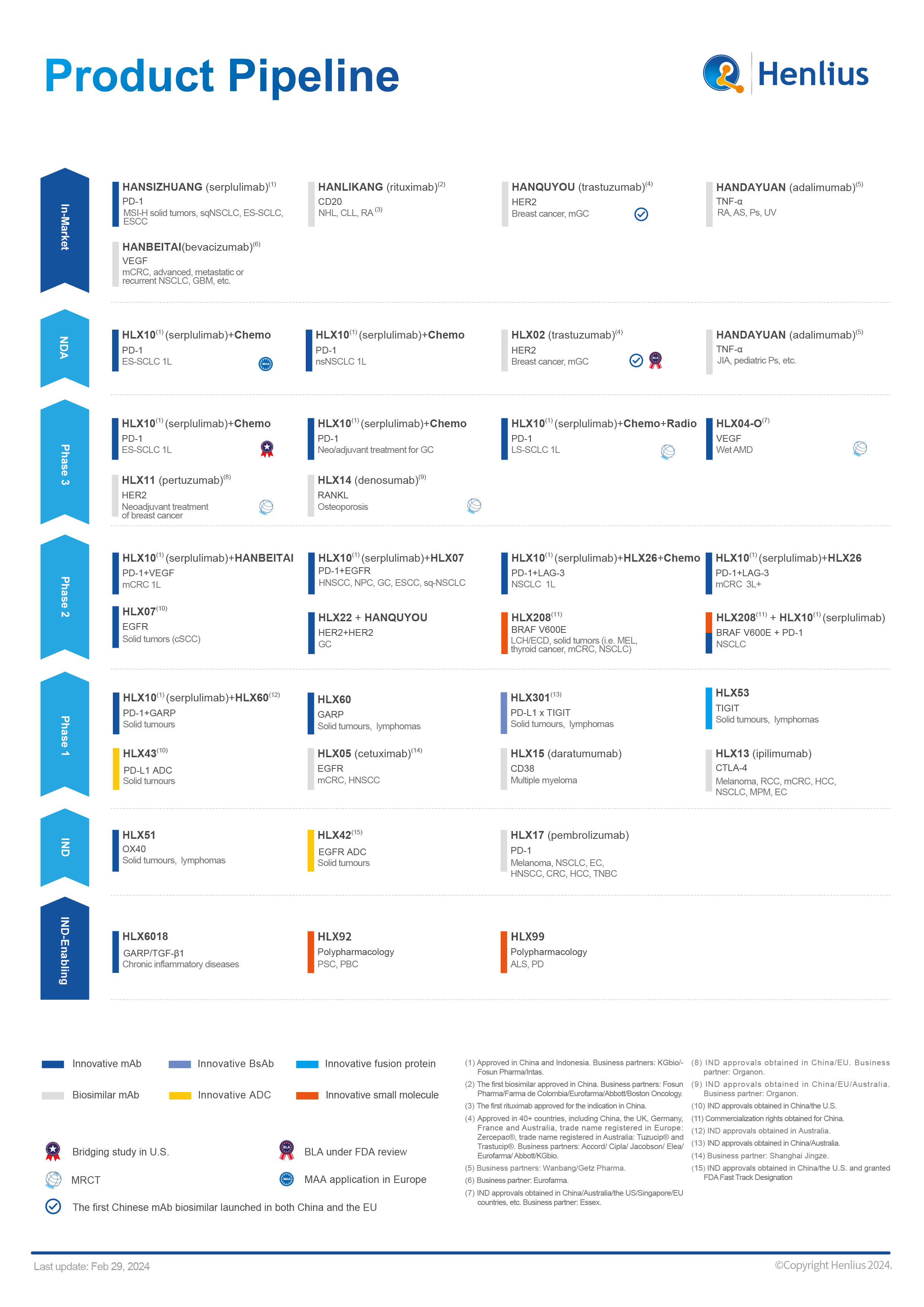
At present, Henlius has launched 5 products and 19 indications, and 7 marketing applications have been accepted for review in China, the U.S., and the EU, respectively. Meanwhile, Henlius has conducted over 30 clinical studies for 16 products globally. HANLIKANG (rituximab injection), the first product of Henlius, has been approved by the National Medical Products Administration (NMPA) as China's first biosimilar. The second product HANQUYOU (trastuzumab, Zercepac® in the EU,Trade names in Australia: Tuzucip® and Trastucip®) is the first Chinese mAb biosimilar approved both in the EU and China, bringing more treatment options to patients with HER2 positive breast and gastric cancer worldwide. In March 2022, the company’s first self-developed innovative monoclonal antibody HANSIZHUANG (serplulimab injection), was launched. It has been approved by the NMPA for the treatment of Microsatellite Instability-High (MSI-H) solid tumors and squamous non-small cell lung cancer (sqNSCLC) and extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC).